Abstract
BACKGROUND Patients affected by β-Thalassemia experience increased muscular fatigue and lower tolerance to physical effort, which considerably contribute to the overall burden of the disease. However, the pathophysiological mechanisms involved in these manifestations are still poorly elucidated. Until now, the higher fatigability observed in these patients has been related to lower hemoglobin levels, lower cardiac efficiency, increased deconditioning, or a combination of these factors. Challenging this hypothesis, we present here a study of neuromuscular function in β-Thalassemia patients, evaluating its possible association with chronic tissue hypoxia, which is known to affect the neuromuscular systems in other models and conditions.
METHODS This is a cross-sectional study evaluating neuromuscular, hematological, biochemical and clinical features of patients affected by Transfusion-Dependent β-Thalassemia (TDT) or Non Transfusion-dependent β-Thalassemia (NTDT) receiving optimal standard of care (SoC), compared to healthy subjects. High-density surface EMG signals were recorded from the vastus medialis and vastus lateralis under isometric contractions at 30% (for 10 s) and 60% (until exhaustion) of maximal voluntary torque. Average muscle fiber size was estimated by muscle fiber conduction velocity (MFCV) and neural drive by motor unit firings. Serum HIF1α and HIF2α were measured by ELISA; hematological and biochemical parameters were measured by routinary hospital lab procedures; clinical data were obtained by clinical records. GPAQ and modified FACIT-F scores assessed the overall physical activity and the quality of life (QoL), respectively. The study was approved by the local EC/IRB.
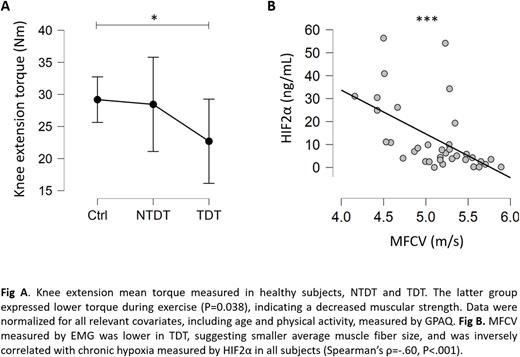
RESULTS A total of 36 subjects (15 healthy, 10 NTDT, 11 TDT) were enrolled in the study. Median age was 29 (range 20-43), 50% were female. Knee extension mean torque was lower in TDT group (P=0.038), controlled for all relevant covariates, including GPAQ. When controlling for recruitment threshold and discharge rate at recruitment, the motor unit discharge rates were higher (P=0.014) in TDT (12.0±0.3 Hz) than in healthy controls (10.9±0.2 Hz), while MFCV was lower (P=0.027) in TDT (4.8±0.5 m/s) than in healthy controls (5.3±0.5 m/s). NTDT showed intermediate values for all parameters considered above. Serum HIF1α levels were 4.3 ±4.3, 2.9 ±1.9, and 24.9 ±42.2 ng/mL in healthy, NTDT and TDT, respectively (P=0.056). HIF2α levels did not differ significantly among groups. Both HIF1α and HIF2α correlated negatively with max torque exercised (Spearman's ρ = -0.355, P=0.036 and ρ = -0.416, P=0.013, respectively) and with MFCV (ρ = -0.43, P=0.02 and ρ = -0.60, P<0.001, respectively). Both HIFα isoforms correlated negatively with baseline venous lactate (ρ = -0.47, P=0.031 and ρ = -0.44, P=0.044, respectively). QoL was significantly lower in patients and positively correlated with exercise capacity (P<0.05). No correlation was observed between hemoglobin and any functional parameter.
DISCUSSION β-Thalassemia patients showed lower muscular strength compared to controls, more prominent in TDT than NTDT, as expected, but no significant differences in muscle endurance. Since no differences in physical activity or training were encountered among groups, muscle deconditioning alone could hardly account for such a reduction. Instead, this observation could be explained by the presence of an altered neuromuscular environment, as indicated by the lower muscle fiber conduction velocity, which suggests smaller average muscle fiber size, and by the higher neural drive, which reflects lower neuromuscular efficiency in patients. In this scenario, chronic tissue hypoxia observed at baseline (measured by HIF1α and HIF2α) could represent a key factor to promote pathological modification of the neuromuscular microenvironment in thalassemic patients, as suggested by the negative correlations among HIF1α, HIF2α and parameters of neuromuscular performance. For these reasons, we suggest chronic tissue hypoxia to be a possible significant contributor to the overall burden of the disease, even in the presence of an optimal treatment received according to the best SoC. To our knowledge, this represents the first observation of such an association in this setting. Further studies should address the molecular mechanisms responsible for this alteration and evaluate any possible target for treatment.
Disclosures
Longo:Celgene: Honoraria; Vertex: Membership on an entity's Board of Directors or advisory committees. Piga:Celgene: Honoraria; Chiesi: Honoraria. D'Avolio:Jannsen: Consultancy; Waters: Consultancy; Witt: Consultancy; HDC: Consultancy; Nordic Pharma: Consultancy; Merck-Millipore: Honoraria; Novartis: Honoraria; Gilead: Consultancy; Shimadzu: Consultancy; CoQua Lab: Consultancy, Current holder of stock options in a privately-held company, Research Funding; Angelini: Consultancy, Honoraria; Correvio: Research Funding. Ferrero:Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Forma therapeutics: Membership on an entity's Board of Directors or advisory committees; Vertex Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal